
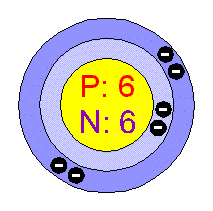
The elements change as you move across the table from block to block, left to right, because you are changing the number of protons in each new block. The atomic number of carbon is six, which means that a carbon atom has six protons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The new atom is an atom of potassium instead. Carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. If you add a proton to a carbon atom, you will not have carbon anymore - it will be nitrogen! If you take a proton away from a calcium atom, it will not be calcium anymore. This is very important to know, because it is the number of protons in the nucleus that determines what element you are working with. Every atom of lithium contains three protons in its nucleus. Every atom of helium has two protons in its nucleus. For example, every atom of hydrogen has one proton in its nucleus - always. What determines the atom carbon In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms) the number of protons in an atom is called the atomic number. See how the atomic number increases by one for each box as you move across the table in a period? This means that each element has its own special number of protons in each atom. That means a carbon atom has 6 protons, 6 neutrons, and 6 electrons. It represents the number of protons found in the nucleus of one atom of that particular element. Carbon on Earth is constantly recycled via the Carbon Cycle. The element forms in stars via the triple-alpha process in which helium atoms fuse to form atomic number 4 (beryllium), which then fuses with atomic number 2 (helium) to form atomic number 6.
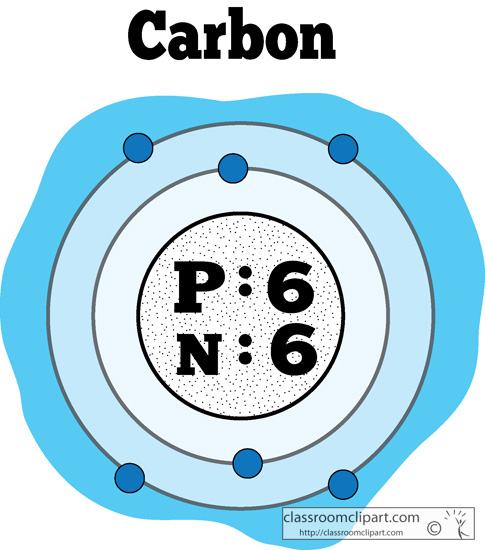
That is, it is possible to determine the properties of carbon from the electron configuration. Carbon is the fourth most abundant element in the universe. Now the electron configuration of carbon shows that the last orbital of carbon has two electrons. This is the atomic number of the element. That is, the atom of the carbon element has a total of six electrons. Carbon 6 12.011 Glossary Group A vertical column in the periodic table. First, there is an integer (whole number) in some part of the box. It has the atomic number 6 in the periodic table and belongs in Group 14. Notice that no matter what form of the table you are using, there are always three very specific items that appear for each element. Carbon (C) exists as several allotropes, most commonly as Diamond or the gray-black solid Graphite.
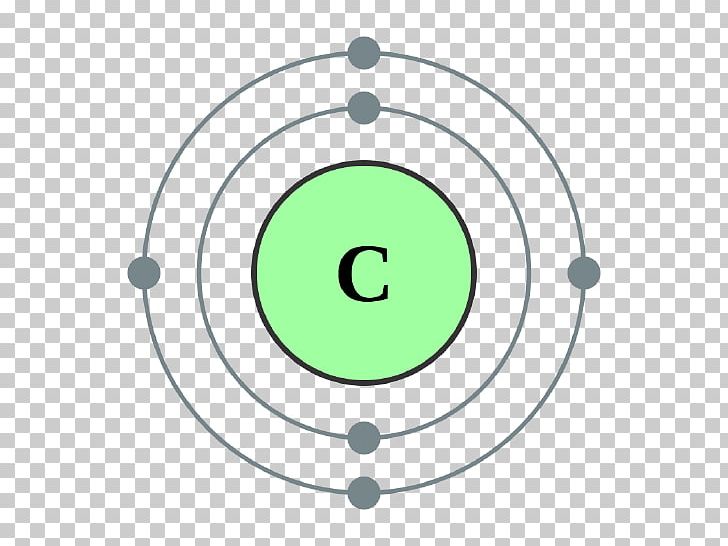
Look very carefully at the boxes in the periodic table.


 0 kommentar(er)
0 kommentar(er)
